Biology DivisionLaboratory of Microbiology
Osada Unit
Research Outline
Osada unit aims to discover new bioactive small molecules that will contribute to the development of novel pharmaceuticals and agrochemicals. We test the effects of screening samples against cancer cells and pathogenic microbes, contributing to discover distinctive anticancer drugs and antibiotics. For the antifungal screening, we have constructed phenotypic screening systems based on new technologies, such as artificial intelligence (AI), heterozygous gene deletion mutant collection, and silkworm infection models.
Members
Please replace [at] in the email address with @, the at symbol.
Specially Appointed Director Hiroyuki Osada osadah[at]bikaken.or.jp
| Graduate Student | Hiro Sakuma |
| Graduate Student | Haruna Yoshimoto |
| Visiting Researcher | Yushi Futamura |
| Visiting Researcher | Harumi Aono |
Themes
Theme outlines
1. Phenotypic screening for new antifungal agents
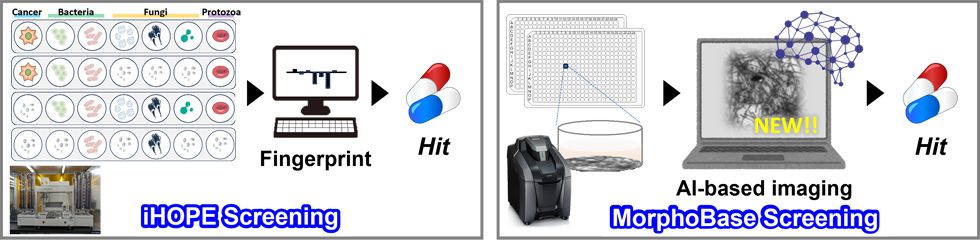
We carry out in-house phenotypic evaluation (iHOPE) screening system, where the growth inhibitory activity of test samples against not only fungi, but also mammalian cells, bacteria, and protozoa are systematically evaluated. By profiling characteristic growth inhibition patterns, called “Fingerprints”, highly specific antifungals can be selected. We have also constructed other phenotypic screening system using MorphoBase, a database of cell morphological changes induced by typical antifungal drugs, and use MorphoBase to screen promising first-in-class compounds from initial hits. Currently, we are improving the system by introducing AI that has learned a huge amount of image data to accelerate our drug discovery program.
2. Target identification of novel antibiotics
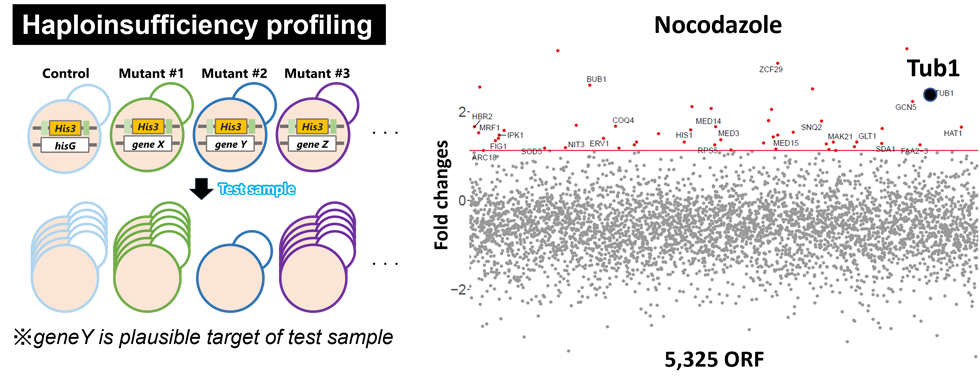
The Merck Candida albicans double barcoded strain library (DBC) is a collection of haploid mutants in ca. 5,000 distinct genes. Haploinsufficiency is a phenomenon where the drug sensitivity increases when the amount of the target protein is reduced. The genome-wide haploinsufficiency profiling using this collection enabled to speculate the mode of action of various antifungal agents (PLoS Pathog, 2007). We are analyzing the mode of action of screening hits by this genetic interaction-based approach as well as other physiological interaction-based ones, such as affinity selection by compound-immobilized beads and cell thermal shift assay.
3. in vivo Antifungal activity test using silkworms as a model organism
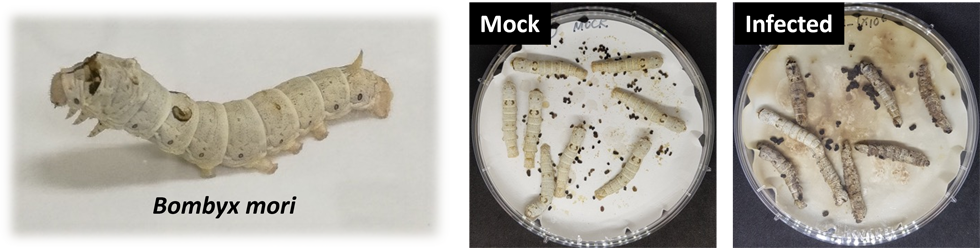
Compared to mice as a model organism, silkworms have many advantages, such as easy to handle, low cost, and few ethical issues. Recently, in vivo models using silkworms infected with various pathogenic microbes were reported and utilized for the evaluation of therapeutic potential of antibiotics (Microbe Pathog, 2002). We applied this system and are evaluating in vivo antifungal activity of the antifungal candidates.
Publications
BIL7 enhances plant growth by regulating the transcription factor BIL1/BZR1 during brassinosteroid signaling.
T. Miyaji, A. Yamagami, Y. Nakamura, K. Nishida, R. Tachibana, S. Surina, S. Fujioka, M. Garcia-Hourquet, S. Mora-García, S. Nosaki, T. Miyakawa, M. Tanokura, M. Matsui, H. Osada, K. Shinozaki, T. Asami, and T. Nakano.
Plant J, 2024, doi: 10.1111/tpj.17212
Synergistic involvement of the NZF domains of the LUBAC accessory subunits HOIL-1L and SHARPIN in the regulation of LUBAC function.
Y. Toda, H. Fujita, K. Mino, T. Koyama, S. Matsuoka, T. Kaizuka, M. Agawa, S. Matsumoto, A. Idei, M. Nishikori, Y. Okuno, H. Osada, M. Yoshida, A. Takaori-Kondo, and K. Iwai.
Cell Death Dis, 2024, 15, 813, doi: 10.1038/s41419-024-07199-z
Reprogramming of flagellin receptor responses with surrogate ligands.
D. H. Lee, H. S. Lee, M. S. Choi, K. Parys, K. Honda, Y. Kondoh, J. M. Lee, N. Edelbacher, G. Heo, B. Enugutti, H. Osada, K. Shirasu, and Y. Belkhadir.
Nat Commun, 2024, 15, 9811, doi: 10.1038/s41467-024-54271-5
Total synthesis, stereochemical assignment, and biological evaluation of opantimycin A and analogues thereof.
Y. Usuki, R. Abe, K. Nishiguchi, T. Satoh, H. Aono, T. Nogawa, Y. Futamura, H. Osada, I. Yoshida, K. Fujita, T. Mishima, and K. I. Fujita.
Org Biomol Chem, 2024, 22, 8973-8979, doi: 10.1039/d4ob01475h
Modulation of fungal phosphate homeostasis by the plant hormone strigolactone.
J. M. Bradley, M. Bunsick, G. Ly, B. Aquino, F. Z. Wang, D. Holbrook-Smith, S. Suginoo, D. Bradizza, N. Kato, O. As'sadiq, N. Marsh, H. Osada, F. D. Boyer, C. S. P. McErlean, Y. Tsuchiya, R. Subramaniam, D. Bonetta, P. McCourt, and S. Lumba.
Mol Cell, 2024, 84, 4031-4047, doi: 10.1016/j.molcel.2024.09.004
Pyricularia oryzae enhances Streptomyces griseus growth via non-volatile alkaline metabolites.
R. Sugiura, T. Arazoe, T. Motoyama, H. Osada, T. Kamakura, K. Kuramochi, and Y. Furuyama.
Environ Microbiol Rep, 2024, 16, e70012, doi: 10.1111/1758-2229.70012
Porphyrin derivatives inhibit tumor necrosis factor α-induced gene expression and reduce the expression and increase the cross-linked forms of cellular components of the nuclear factor κB signaling pathway.
Q. V. Vu, N. T. Vu, K. Baba, S. Sasaki, R. Tamura, K. Morimoto, H. Hirano, H. Osada, and T. Kataoka.
Eur J Pharmacol, 2024, 977, 176747, doi: 10.1016/j.ejphar.2024.176747
Structural and functional analyses of inhibition of human dihydroorotate dehydrogenase by antiviral furocoumavirin.
M. Nakahara, S. Watanabe, M. Sato, H. Okumura, M. Kawatani, H. Osada, K. Hara, H. Hashimoto, and K. Watanabe.
Biochemistry, 2024, 63, 1241-1245, doi: 10.1021/acs.biochem.4c00120
Identification of a family of species-selective complex I inhibitors as potential anthelmintics.
T. Davie, X. Serrat, L. Imhof, J. Snider, I. Štagljar, J. Keiser, H. Hirano, N. Watanabe, H. Osada, and A. D. Fraser.
Nat Commun, 2024, 15, 3367, doi: 10.1038/s41467-024-47331-3
Expression of Syo_1.56 SARP regulator unveils potent elasnin derivatives with antibacterial activity.
I. A. Abdelhakim, Y. Futamura, Y. Asami, H. Hanaki, N. Kito, S. Masuda, A. Shibata, A. Muranaka, H. Koshino, K. Shirasu, H. Osada, J. Ishikawa, and S. Takahashi.
J Nat Prod, 2024, 87, 1459-1470, doi: 10.1021/acs.jnatprod.4c00259
Reclassification of a reveromycin-producer and the proposals of Actinacidiphila reveromycinica sp. nov. and Actinacidiphila acidipaludis comb. nov.
H. Komaki, H. Takagi, S. Takahashi, and H. Osada.
Biosci Biotechnol Biochem, 2024, 88, 689-695, doi: 10.1093/bbb/zbae032
Production of kinanthraquinone D with antimalarial activity by heterologous gene expression and biotransformation in Streptomyces lividans TK23.
K. Sakai, Y. Futamura, T. Nogawa, Y. Zhao, H. Koshino, H. Osada, and S. Takahashi.
J Nat Prod, 2024, 87, 855-860, doi: 10.1021/acs.jnatprod.3c01076
Amiodarone inhibits the Toll-like receptor 3-mediated nuclear factor κB signaling pathway by blocking organelle acidification.
Y. Yokota, K. Takaki, K. Baba, S. Sasaki, H. Hirano, H. Osada, and T. Kataoka.
Biochem Biophys Res Commun, 2004, 708, 149801, doi: 10.1016/j.bbrc.2024.149801
Alantolactone derivatives inhibit the tumor necrosis factor α-induced nuclear factor κB pathway by a different mechanism from alantolactone.
Q. V. Vu, K. Baba, S. Sasaki, K. Kawaguchi, H. Hirano, H. Osada H, and T. Kataoka.
Eur J Pharmacol, 2024, 969, 176458, doi: 10.1016/j.ejphar.2024.176458

 +81-3-3441-4173
+81-3-3441-4173  office@bikaken.or.jp
office@bikaken.or.jp