Chemistry DivisionLaboratory of Chemistry
Research Outline
Development of asymmetric catalysts with novel concepts to produce optically active compounds.
Synthetic studies on biologically active natural products.
Members
Please replace [at] in the email address with @, the at symbol.
Laboratory Head Takumi Watanabe twatanabe[at]bikaken.or.jp

Chief Researcher Hidetoshi Noda hnoda[at]bikaken.or.jp

Senior Researcher Kazushige Sasaki sasakik[at]bikaken.or.jp

Senior Researcher Toshifumi Takeuchi takeuchit[at]bikaken.or.jp
| Researcher | Hikaru Abe |
| Researcher | Raphael Oriez |
| Researcher | Yasunari Otsuka |
| Researcher | Akira Saito |
| Researcher | Atsushi Seki |
| Researcher | Hisashi Takada |
| Postdoctoral Researcher | Sadhanendu Samanta |
| Postdoctoral Researcher | Manoharan Ramasamy |
| Graduate Student | Makiko Komagata |
| Graduate Student | Shunsuke Watanabe |
Two more Technical Staff
Themes
Theme outlines
1. Medicinal chemistry based on natural products
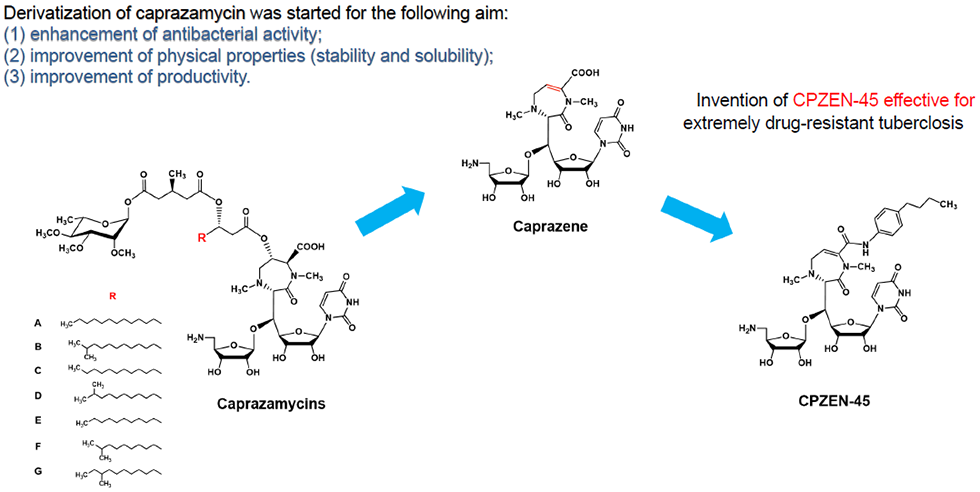
2. Development of methodology to produce optically active compounds
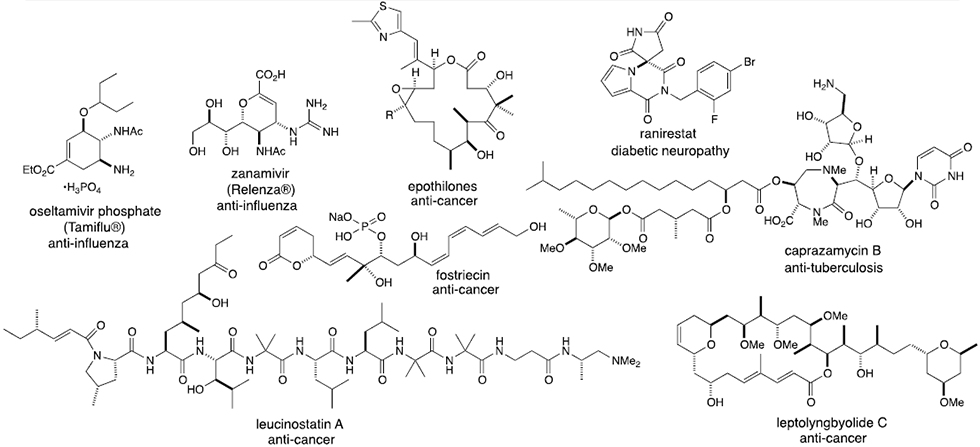
3. Applications of catalytic asymmetric reactions to various biologically active natural products and therapeutics

 +81-3-3441-4173
+81-3-3441-4173  office@bikaken.or.jp
office@bikaken.or.jp