Prionase
- Project Leader, Hiroyasu Doi
- E-mail; doih@bikaken.or.jp
- Yuichi Kasahara (technician)
- Ikuko Osawa (assistant)
- Takako Mawatari (assistant)
Backgrounds of research
Bovine spongiform encephalopathy (BSE), also known as mad cow disease, is caused by infectious misfolded prion proteins and has been regarded as an epidemic problem in recent years. “Prion diseases” is the collective term for diseases caused by abnormal prion proteins that have been converted from and have different conformation to normal cellular prions. Such diseases are found in humans and a number of animal species. Examples include scrapie in sheep, and kuru, Creutzfeldt-Jakob disease, Gerstmann-Straussler-Scheinker syndrome, and fatal familial insomnia in humans.
Normal prion protein is expressed in normal brain tissue but appears to be converted by an unknown mechanism into a β-sheet-rich isoform, which aggregates to trigger nervous system diseases (Fig. 1). Unlike normal prion protein, abnormal prion protein is (1) strongly resistant to heat, acids, alkalis, detergents, and enzymatic proteolysis, and (2) infectious.
We have collaborated with the late Dr. Tatsuzo Oka, Department of Veterinary Medicine, Faculty of Agriculture, Kagoshima University, Japan and discovered prionase (PRIONASE: Japan trademark nos. 4932402 and 4925193, and USA trademark no. 76642159) in 2004. Prionase is a proteolytic enzyme that degrades abnormal prion proteins to inactivate them. We are now investigating whether prionase can be utilized for the following applications: cleaning and disinfection of instruments used in a slaughterhouse; degradation of abnormal prion proteins in cattle feed containing meat and bone meal; and neutralization of natural sausage casings (e.g., sheep intestine) that are most likely to contain abnormal prion proteins if prepared from an infected animal.
Research accomplishments
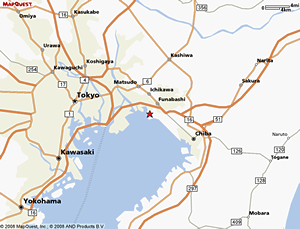
Isolation of a microbial strain producing prionase –– We isolated an actinomycete, inferred as a new Streptomyces strain, from samples collected from the Yatsuhigata tidal flat in Narashino City, Chiba Prefecture, Japan after obtaining research permission in 1999. Yatsuhigata is a remnant of the coastal mudflat developed in the inner part of Tokyo Bay and contains mud rich in organic matter. In addition, 2 canals connect the mud flat to the sea, which results in high levels of biodiversity and organic matter decomposition.
view
The area around Yatsuhigata is reclaimed land.

The 99-GP-2D-5 strain was isolated from water collected in the gateway section of the canal flowing into the tidal flat. At this gateway section, a strong tidal sea water flow repeatedly meets with the water flowing from inland. In addition, there is a large influx of domestic effluent from the surrounding residential areas.

An area of coastal mudflat has turned into an inland mudflat following a series of land reclamation. Yatsuhigata is a designated site under the Ramsar Convention.
In addition to the 99-GP-2D-5 strain, several other microorganisms exhibiting strong proteolytic activities were isolated from the samples collected in Yatsuhigata.
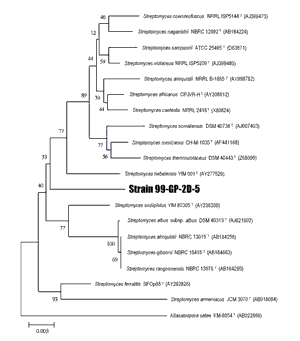
Prionase-producing microorganism strain 99-GP-2D-5 –– The 99-GP-2D-5 strain is an actinomycete that was inferred from a phylogenetic tree based on 16S ribosomal DNA sequences, and is a member of the genus Streptomyces (Figs. 5 and 6).
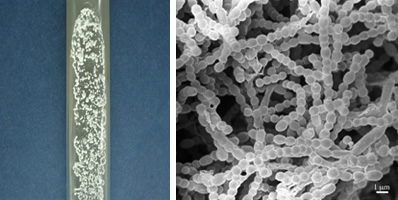
Images showing the colony morphology of the strain 99-GP-2D-5 grown on a slant culture medium (left) and as viewed using an electron microscope (right). Both images were contributed by Ms. Naoko Kinoshita, IMC.
view
Neighbor-joining phylogenic tree showing the relationship between strain 99-GP-2D-5 and related species of the genus Streptomyces on the basis of nearly complete 16S rDNA gene sequences. The scale bar denotes the number of substitutions per nucleotide position. The numbers on the branches indicate the percentage bootstrap values. Each bar represents 0.005 substitutions per nucleotide position.
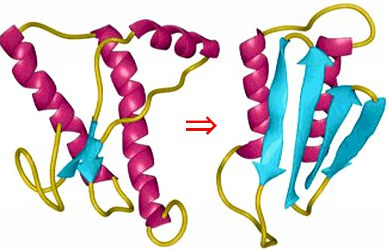
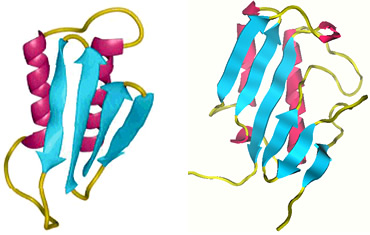
The search for prionase –– Due to a risk of transmission, research on abnormal prion proteins can only be performed in laboratories with a biosafety level of P3 or higher. Therefore, a certain level of practical difficulty exists. In addition, since the incidence of mad cow disease was low in Japan at the early stage of the project, we had difficulties in obtaining abnormal prion proteins. With this background, we used perchloric acid-soluble protein (PSP), which has a similar conformation to the abnormal prion protein and exhibits resistance against heat, acids, alkalis, and enzymatic proteolysis, as an alternative substrate. PSP was discovered in the liver by Oka et al. in 1995 as an inhibitor of protein synthesis. It is an mRNA-specific nuclease composed of six inner β-strands and two outer α-helices (Fig. 7), and is implicated in suppression of immunological diseases and anti-cancer action. Prionase was found after screening more than 1,000 microbe cultures using PSP degradation as an index (Fig. 8).
Fig. 7 Comparison of conformations between the abnormal prion protein (left) and perchloric acid-soluble protein (right)

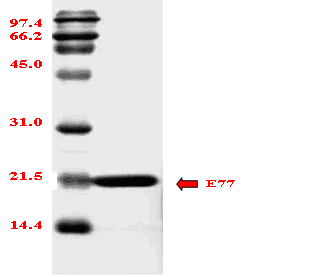
Structure and characteristics of prionase –– Prinoase is a novel proteolytic enzyme with a molecular weight of approximately 19 kDa, and functions optimally at a temperature of 70℃ and a pH of 8.
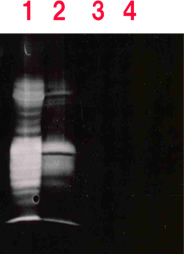
1= Control
2= Treated with proteinase K
3= 10 µL of prionase containing broth (30 min 37℃)
4= 20 µL of prionase containing broth (30 min 37℃)
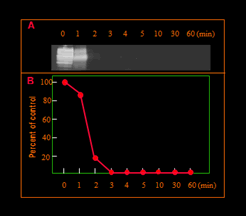
A: Western blot of the digests of scrapie prion protein
B: Percentages of the amount of remaining scrapie prions at each prionase treatment applied to the initial sample (time 0).
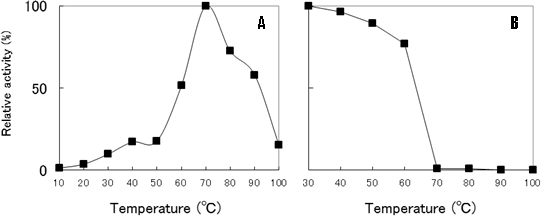
Fig. 12 Effects of temperature on prionase activity (A) and stability (B) of E77. Protease stability (B) was measured at 37℃ for 20 min following incubation at temperature increments of 10℃ for 1 hour.
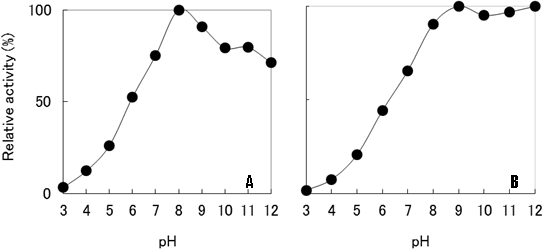
Fig. 13 Effects of pH on prionase activity (A) and stability (B) of E77
Anticipated accomplishments
-
Cleaning and disinfection of instruments used in medical settings and slaughterhouses
Abnormal prion protein can only be inactivated by incineration. Conventional disinfection procedures (e.g., autoclaving) performed in general medical institutions are ineffective. However, the use of prionase-containing disinfectant can completely degrade abnormal prion proteins.
-
Treatment of effluents contaminated with abnormal prion proteins
It is possible that abnormal prion proteins in soil, groundwater, and surface reservoirs can initiate a new transmission cycle when released into the environment. Prionase can completely degrade abnormal prion proteins, thereby neutralizing the pathogen and consequently eliminating the risk of re-infection of humans and animals, and further transmission of the pathogens to fertilizers and crops. Abnormal prion proteins can be completely degraded by mild incubation with prionase at 37℃ for 10 min, while more intense conditions (134℃, 18 min) are required for inactivating infectious prions by autoclaving.
-
Safe use of meat and bone meal by prionase treatment
A vast amount of meat and bone meal is generated as industrial waste by the livestock industry. This waste is a mixture of inedible meat, brain, spinal cord, bones, internal organs, and blood of bovines, pigs, and chickens. The production procedure includes several processes such as heating, delipidation, drying, and milling. Such waste is used to produce feed for cattle (e.g., pigs and chickens and pets, as well as fertilizers and cement materials. In this application, prionase is expected to play an important role for the safe use of meat and bone meal.
-
Use of prionase in the food industry
Sheep intestines are used as natural sausage casings. Incidentally, an experimental transmission of BSE to sheep was confirmed in France. However, a case of natural transmission of BSE has not yet been reported. As the French incident suggested the possible use of BSE-infected sheep intestines for sausage casings, the food industry should safeguard itself with a technique for removing the prion proteins that cause BSE and scrapie. In a collaborative research project conducted with Kagoshima University and Matsunaga Corporation, we found that treatment of prionase by incubation with prionase at an appropriate temperature, stopping the enzymatic reaction by adding an inhibitor, and deactivation of the enzyme by washing with water, successfully removed abnormal prion protein from contaminated natural casings.
-
Jellyfish decomposition by prionase
As prionase is a strong proteolytic enzyme, it is also useful for the treatment of other animal proteins resistant to proteolytic decomposition. We recently developed a system enabling the complete decomposition of landed jellyfish near power plants, and other jellyfish including Nemopilema nomurai Kishinouye, which are known to cause serious damage along the shores of the Sea of Japan. The system requires only 30 min for the complete decomposition of jellyfish proteins. The practical use of this prionase-utilizing system is currently in the final stage of evaluation.

Fig. 14 Nomura’s jellyfish (Nemopilema nomurai Kishinouye) trapped in a fixed net
The images were kindly provided by Mr. Shuzaburo Hirakawa, the owner of No. 18 Yokoh Maru, Oga City, Akita Prefecture, Japan.
Related publications
Related patents (international)
Presentation in international conferences (Last five years)
- Alkaline serine protease produced by Streptomyces sp. Degrades PrPsc.
M. Okabe, Zhao H., H. Doi, Y. Matsuura, S. Mouri, Y. Nonomura, H. Kanouch and T. Oka
10th Asian congress of nutrition Taipei international convention center Taipei, Taiwan, Sep. 9-13, 2007 - Screening of PrPsc-degrading enzyme and its inhibitor.
M. Okabe, Zhao H. Doi, Y. Matsuura, S. Mouri, Y. Nonomura, H. Kanouchi and T. Oka
ICPH2007 satellite symposium in Kagoshima-food and health in Asia-Kagoshima Japan, Nov. 23-24, 2007