Publication --> 2020

"Design, Synthesis, and Application of Multiboron Heterocycle to Direct Amidation Catalyst"
Noda, H.; Shibasaki, M.; Kumagai, N.
J. Synth. Org. Chem. Jpn. 2020, 78, 971.

"Direct Catalytic Asymmetric Addition of α‐Fluoronitriles to Aldehydes"
Balaji, P. V.; Li, Z.; Saito, A.; Kumagai, N.; Shibasaki, M.
Chem. Eur. J. 2020, 26, 15524.
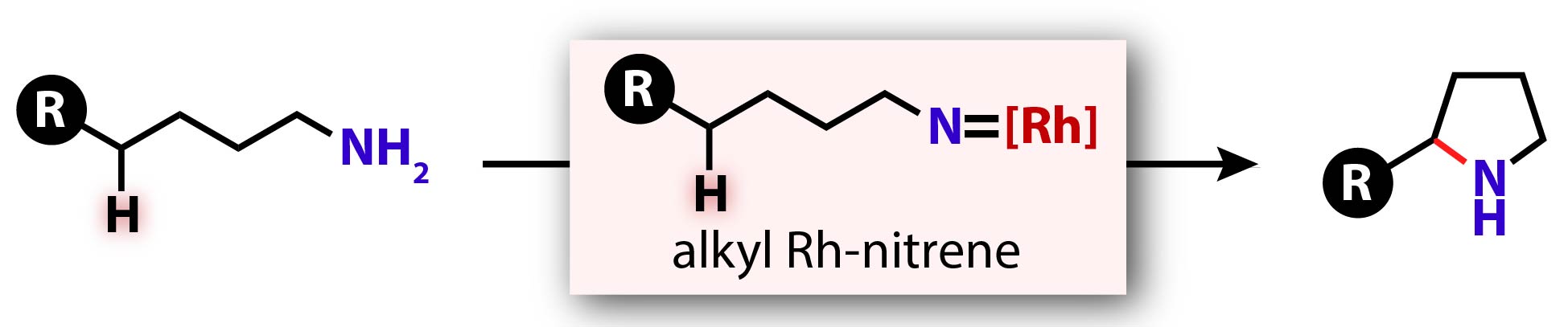
"O-Benzoylhydroxylamines as Alkyl Nitrene Precursors: Synthesis of Saturated N-Heterocycles from Primary Amines"
Noda, H.; Asada, Y.; Shibasaki, M.
Org. Lett. 2020, 22, 8769.
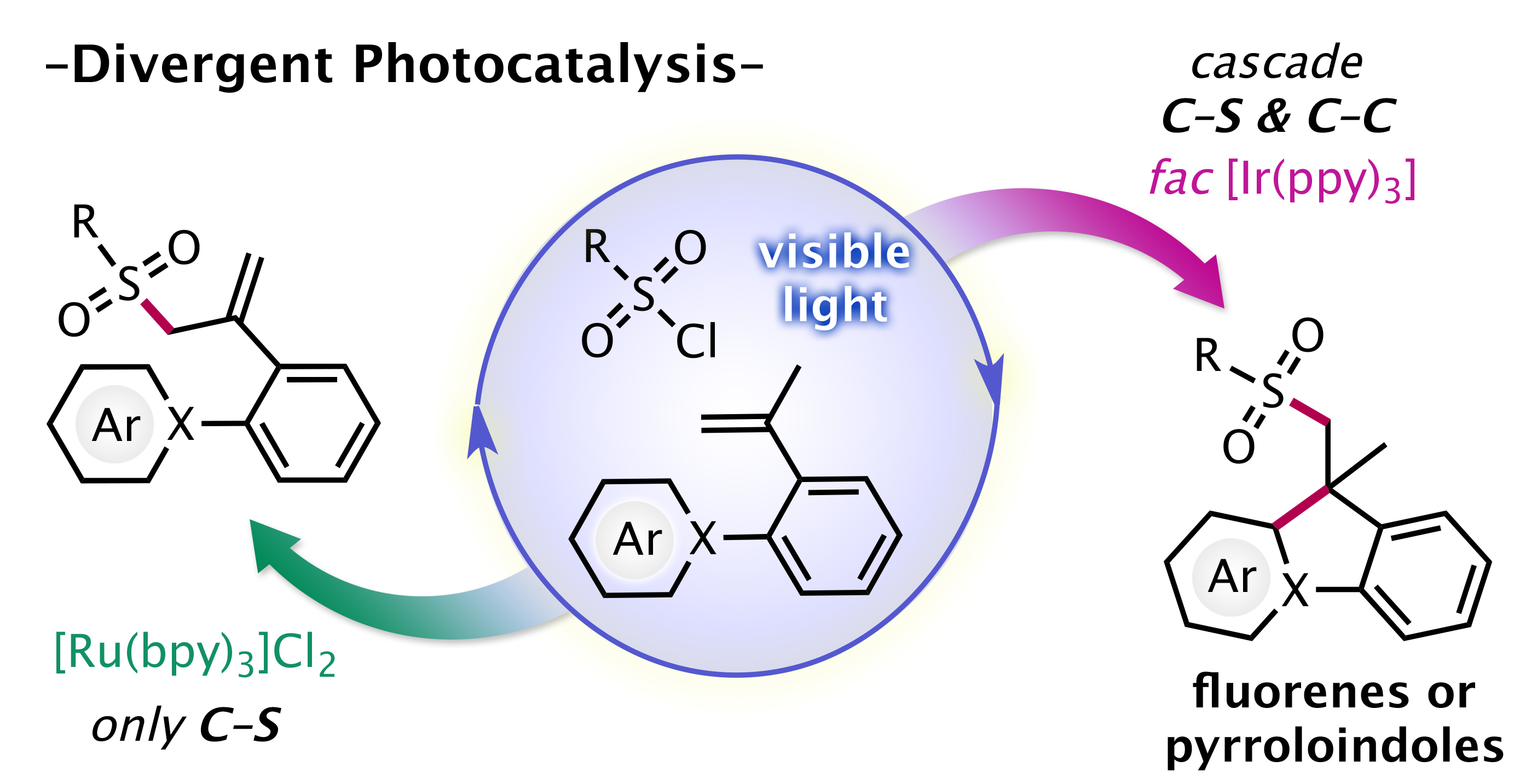
"The Different Faces of [Ru(bpy)3Cl2] and fac[Ir(ppy)3] Photocatalysts: Redox Potential Controlled Synthesis of Sulfonylated Fluorenes and Pyrroloindoles from Unactivated Olefins and Sulfonyl Chlorides"
Pagire, S. K.; Kumagai, N.; Shibasaki, M.
Org. Lett. 2020, 22, 7853.
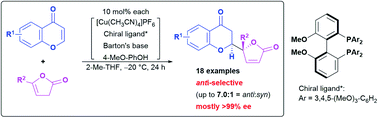
"Direct catalytic asymmetric and anti-selective vinylogous addition of butenolides to chromones"
Cui, J.; Kumagai, N.; Watanabe, T.; Shibasaki, M.
Chem. Sci. 2020, 11, 7170. Highlighted in Synfacts 2020, 11, 7170.
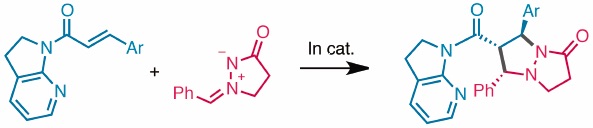
"Catalytic Asymmetric 1,3-Dipolar Cycloaddition of α,β-Unsaturated Amide and Azomethine Imine"
Li, Z.; Kumagai, N.; Shibasaki, M.
Chem. Pharm. Bull. 2020, 68, 552.
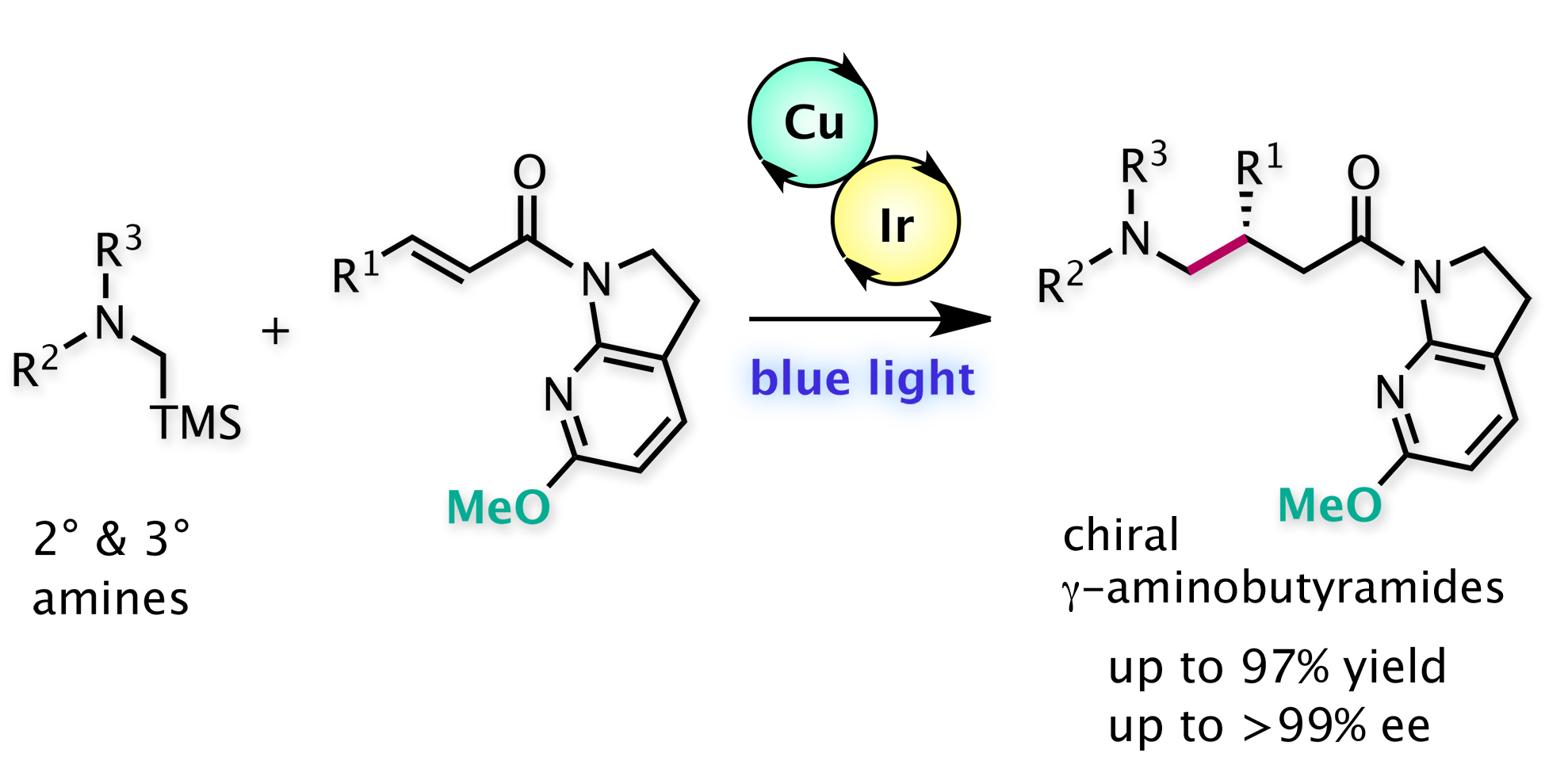

"Introduction of a 7-aza-6-MeO-indoline auxiliary in Lewis-acid/photoredox cooperative catalysis: highly enantioselective aminomethylation of α,β-unsaturated amides"
Pagire, S. K.; Kumagai, N.; Shibasaki, M.
Chem. Sci. 2020, 11, 5168. Selected as front cover.
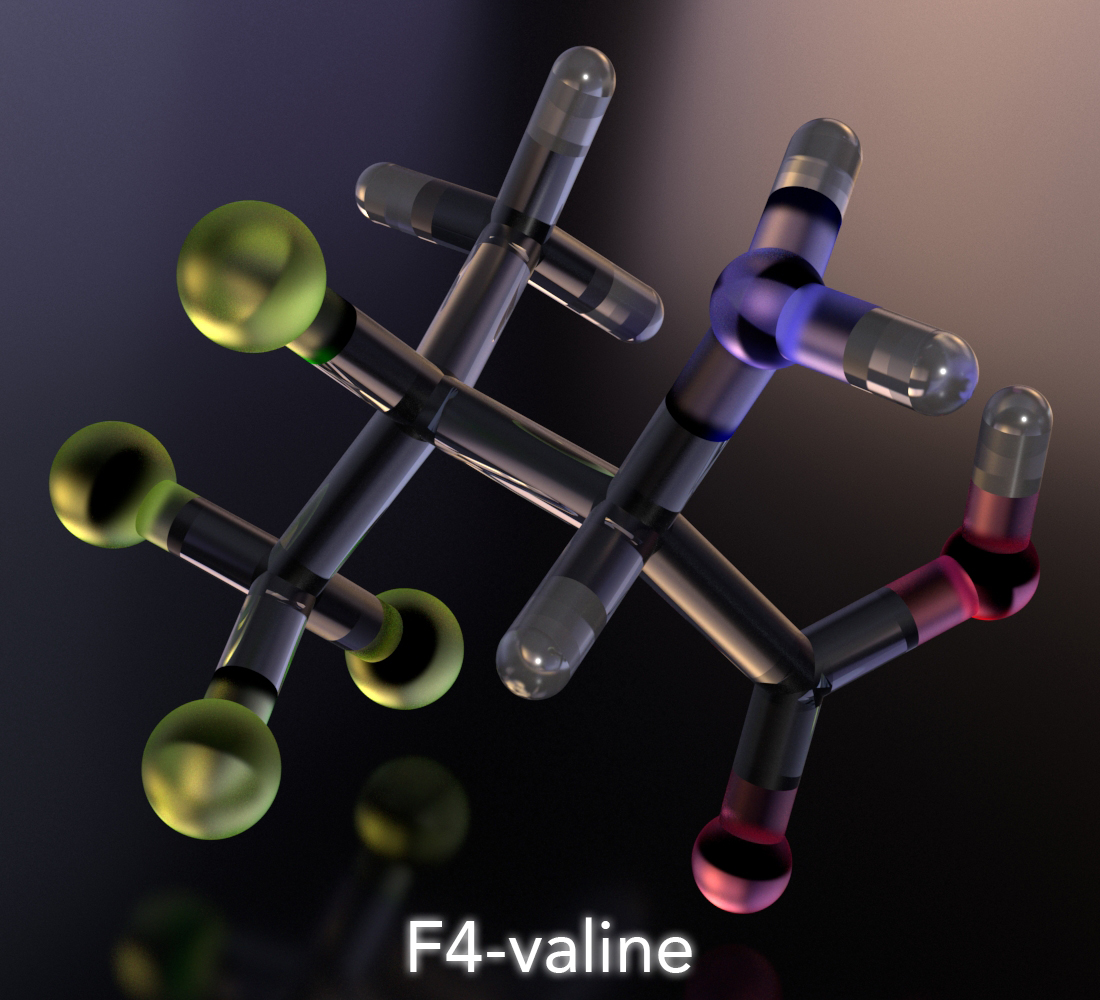
"(2R,3S)‐3,4,4,4‐Tetrafluorovaline: A Fluorinated Bioisostere of Isoleucine"
Brewitz, L.; Noda, H.; Kumagai, N.; Shibasaki, M.
Eur. J. Org. Chem. 2020, 1745.
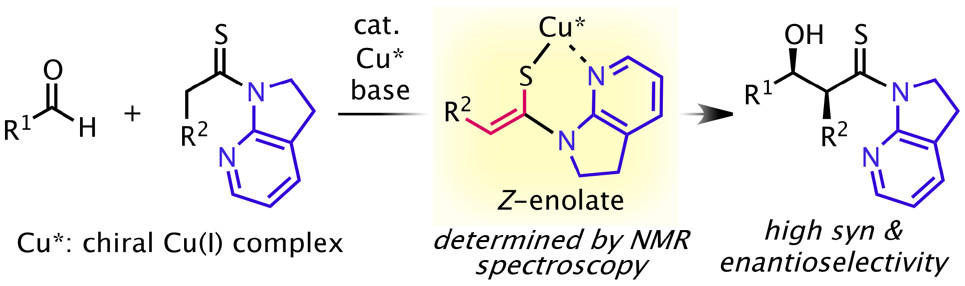

"Z-Enolate Geometry in the Thioamide Aldol Reaction Illuminated by the 7-Azaindoline Auxiliary"
Pluta, R.; Li, Z.; Kumagai, N.; Shibasaki, M.
Org. Lett. 2020, 22, 791.
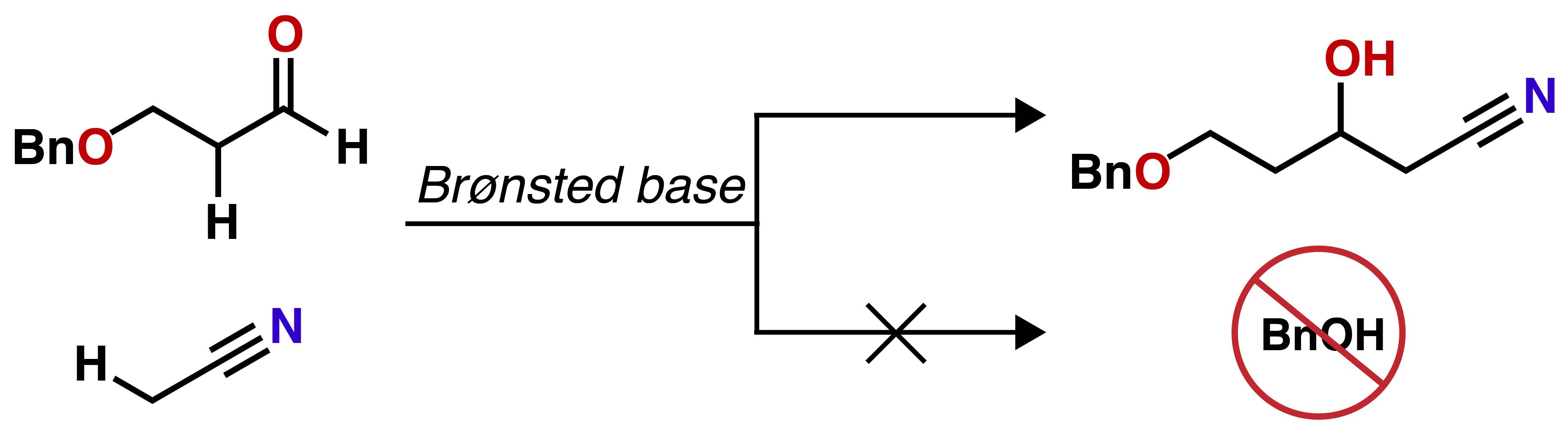

"Cyanomethylation of β‐Alkoxyaldehydes: Toward a Short Synthesis of Atorvastatin"
Tak, R. K.; Noda, H.; Shibasaki, M.
Asian J. Org. Chem. 2020, 9, 57. Selected as Cover Picture.
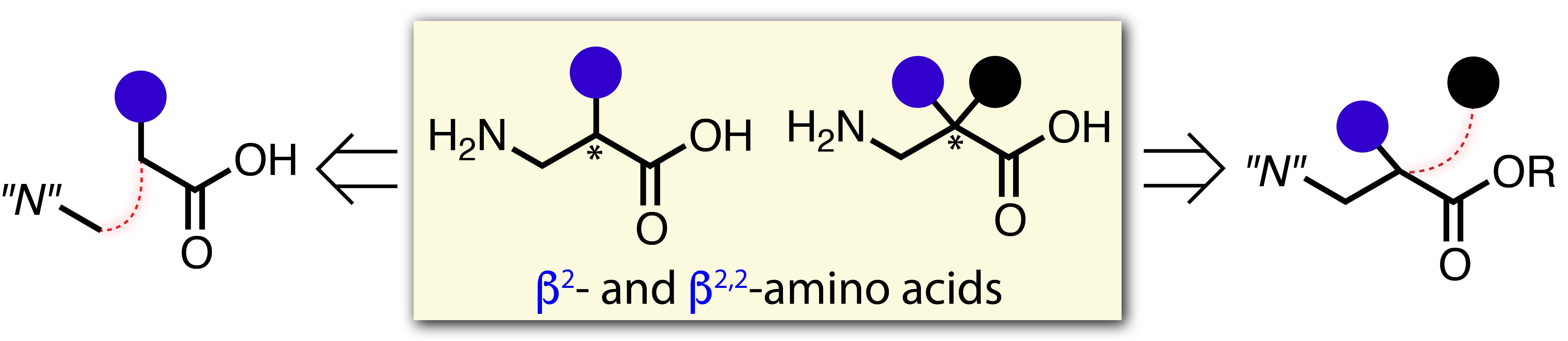
"Recent Advances in the Catalytic Asymmetric Synthesis of β2‐ and β2,2‐Amino Acids"
Noda, H.; Shibasaki, M.
Eur. J. Org. Chem. 2020, 2350.





